Mutation Designer - Overlapping primers for SDM
Our motivation
We are a team of biochemists & molecular biologists, and we found ourselves frequently designing primers for performing several PCR-based whole-plasmid Site-Directed Mutagenesis (SDM) experiments. Designing of SDM primers requires inspection of the codon table and regular monitoring of the dynamically changing primer properties as the primer is being designed. To make our lives a little easier, we wrote Mutation Designer to automate the primer design workflow we currently use in our lab. We hope you'll find Mutation Designer useful.
General information
Using this tool you can generate SDM primers to make completely or partially overlapping SDM primers.
a) Completely overlapping: This strategy of primer design is akin to the Agilent QuickChange™ strategy where the mutation is introduced in the middle of the forward and reverse primers (which are complementary to each other).
b) Partially overlapping: This strategy of primer design is based on Zheng et. al. NAR, 2004 where the improved the PCR amplifiction efficieny of the QuickChange™ SDM protocol by utilizing partially overlapping primers. This protocol completely eliminates the problem associated with primer self-annealing.
As show in the figure below, the desired mutation is introduced by the overlapping primers, both of which carry the mutation. With our tool you can choose whether you want completely overlapping primers or partially overlapping primers (offset by extending 3’ ends as shown in the figure).
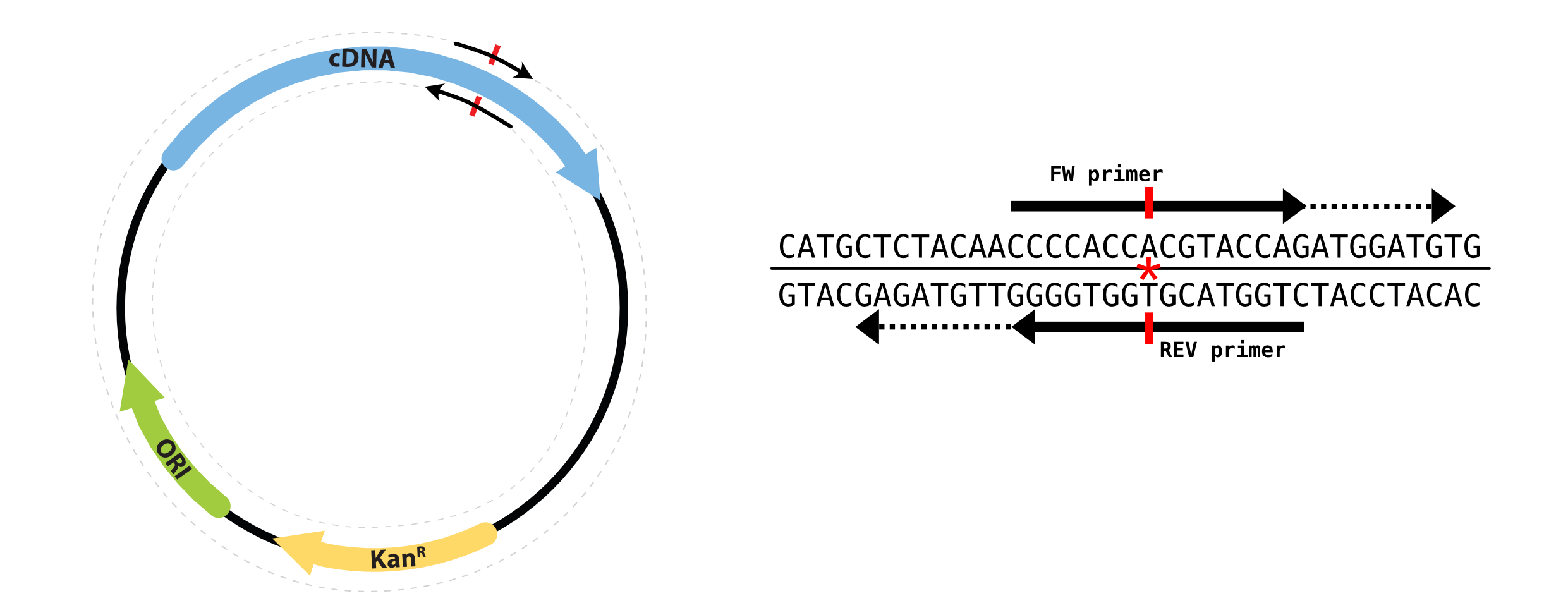
Before transformation the PCR reaction is treated with DpnI restriction enzyme (which cuts methylated DNA, and spares the newly synthesized PCR amplicons) to digest the template DNA.
Mutation designer requires following inputs
- DNA Sequence : A text file with the target DNA sequence as a continuous string of characters.
DNA can be either in upper case, Lower case or even mixed case. DNA can be in several lines (e.g. if it is from a FASTA file), just remember to remove the header from fasta files (i.e. the first line starting with ‘>’)
1
atgaagccggcgacaggactttgggtctgggtgagccttctcgtggcggcggggaccgtccagcccagcgattctcagtcagtgtgtgcaggaacggagaataaactgagctctctctctgacctggaacagcagtaccgagccttgcgcaagtactatgaaaactgt
- List of desired mutations : A text file listing the desired amino acid changes. Each amino acid change should be listed in a new line.
xxxxxxxxxx
5
1
A123T
2
V234Y
3
P456S
4
...
5
... continued
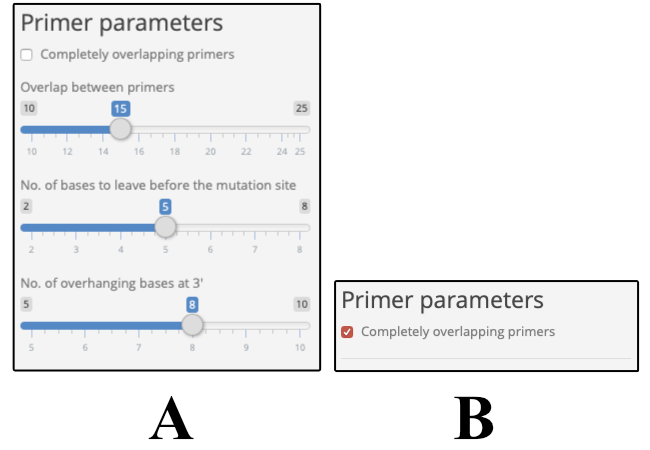
- Complete overlap (checkbox) : To indicate whether you want completely overlapping primers. If this box is checked [panel B in the figure above] the program continues to design primers based on the parameters described in Agilent’s QuickChange manual. In case you want an alteration to the calculations please let us know here, so that we can provide that option with an update to our web-tool.
If the box is left unchecked (default setting), then the user is presented with these following parameters as shown in panel A in the figure above.
- Length of overlap between the primers: Number of nucleotides that are overlapping between the forward and reverse primers. Too long of an overlap increases the likelihood of primer dimer formation.
- Number of bases to leave before the mutation site: Mutation designer tries to introduce the mutation after at least these many bases (while trying to optimize the primer length and incorporating a G/C at 5’ and 3’ end of the primers).
- Number of overhanging bases at 3’ end: Mutation designer tries to leave an overhang at 3’ end which is determined by this option. The overhang can be longer or shorter (based on the Tm and optimizations for ending the primer with a G/C).
Mutation Designer uses nearest neighbor thermodynamics to calculate Tm (melting temperature) which is based on the method described in, Allawi & SantaLucia Jr, Nucleic Acids Res(1998) PMID:9592156.
Output of Mutation Designer
Mutation Designer accepts the above mentioned inputs and predicts primers for a whole-plasmid Site Directed Mutagenesis. Mutation Designer takes these into account the user inputs and attempts to design the "best possible" primer pairs depending on the local genetic context:
-
Primers creating the desired mutation with minimum number of nucleotide changes.
- In the event there are alternative ways to create a point mutation with the same number of nucleotide changes, Mutation designer lists all such possibilities.
-
Primers with a G or C at 5’ and 3' end.
-
Primers overlap decided by the user or automatically based on optimizations (when completely overlapping primers are desired)
-
Primer pairs with a 40 to 60% GC content.
-
Primer pairs with GC content within 15% of each other.
Mutation Designer displays the output to the user on the wepbage on clicking the "Display Results" button.
Although, we strongly recommend clicking the "Download Results" button to obtain a TSV file with a table containing complete information about the predicted primers, including warnings (if any).
Warning Flags of Mutation Designer
There are four warning flags in the output table. Here is how to interpret them:
- Leading_AT_warning is "Y" if the Forward or the Reverse Primer doesn't start with G or C respectively.
- GC_warning_FW is "Y" if the GC content for FW primer is < 40% or >60%.
- GC_warning_REV is "Y" if the GC content for REV primer is < 40% or >60%.
- GC_Diff_Warning is "Y" if the difference in the GC content of the FW and REV primers's is >15%.
In case of primers with warnings, we recommend a close inspection of the nucleotide landscape around the mutation site, and if possible, a re-designing of the primer sequences by altering the input parameters in Mutation Designer or a complete manual re-design (if the results from Mutation Designer are unsatisfactory).
Caveats & Disclaimer:
-
Number of mismatches in the mutagenic primer are not accounted for during the standard Tm calculations.
-
The Tm values are not tailored towards any particular polymerase or protocol. They are merely indicative. Please follow the directions of your PCR kit to calculate the correct annealing temperatures.
-
Like with most PCRs optimization of PCR conditions (Annealing temp, extension time, number of cycles, etc.) might be necessary to get desired results.
-
We are not affiliated with nor do we endorse QuickChange®, Q5®, or any of their competing products and their parent companies. These two products are widely used in their subtype of SDM protocols, therefore we used those names to give you, the user, a quick idea about the differences between our two variants of Mutation Designer.
Points to consider before ordering the oligos
-
Ensure that the forward primer(s) creates your desired amino acid change(s) and the reverse primer matches the anti-sense strand of your template to create a whole plasmid mutagenesis.
-
Check for potential off-target annealing sites for these primers in your host organism by using Primer Blast from NCBI.
-
Check that the primers do not have self-complimentarity and that the primers are not making any secondary structures using the Oligo Analyzer tool by IDT.
Privacy
Being researchers ourselves, we understand the confidentiality of your data, therefore Mutation designer deletes your input files as soon as the execution session ends.
References
- Hatim T. Allawi, John SantaLucia, Jr, Thermodynamics of internal C·T mismatches in DNA, Nucleic Acids Research, Volume 26, Issue 11, 1 June 1998, Pages 2694–2701, https://doi.org/10.1093/nar/26.11.2694
